Metal-air Battery
Inventor/Assignee: Emanuel G Katsoulis, John J Prescia | Assignee: Leesona Corp.
Description:
Introduction: Metal–air batteries (MABs) are a promising technology that could be used in several applications, from portable devices to large-scale energy storage applications. Metal-air batteries are a family of electrochemical cells powered by metal oxidation and oxygen reduction. A metal-air battery is an electrochemical cell that has a metal negative electrode, an air positive electrode, and an electrolyte. The use of oxygen as an active material is beneficial in fabricating high energy battery, material availability, and battery cost. The negative materials are generally base metals such as lithium, Aluminium, and zinc. Primary MABs can be classified into normal (ready-to-use) and reserve batteries whereas secondary MABs can be categorized into electrically rechargeable and mechanically rechargeable types.
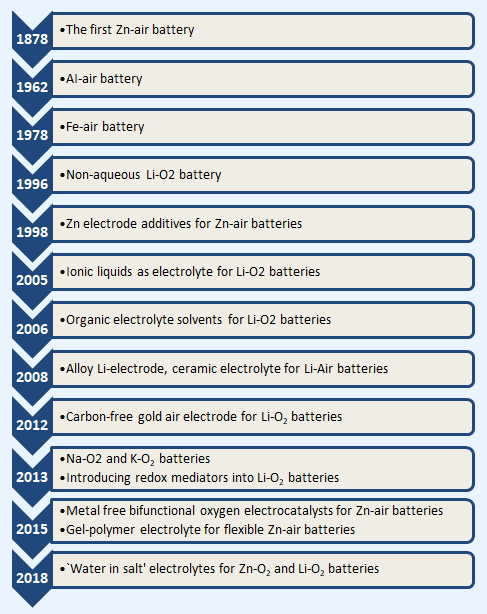
Maturity Timeline:
Advantages: The theoretical energy density of metal-air batteries is about 3–30 times higher than commercial Li-ion batteries.
For Zn-air batteries the theoretical energy density is 1,350 W h kg−1 (based on the mass of Zn metal), still about 5-fold higher than Li-ion batteries. Moreover, Zn-air batteries also have the advantage of low cost (∼$10 kW−1 h−1) compared with Li-ion batteries ($400–800 kW−1 h−1).7 Besides Li-air and Zn-air batteries, other types of metal-air batteries also have their own superiorities. For example, Al-air batteries exhibit the highest volumetric capacity (8,040 A h L−1),8 and Na-air batteries show smaller charge overpotentials than Li-air batteries. Therefore, the metal-air battery family has great potential to serve as next-generation electrochemical energy-storage devices.
Limitations:
- The metal anodes react with the electrolyte to form a passivation layer called solid electrolyte interphase (SEI) film. This film causes an irreversible loss in battery performance.
- The dendrite growth on anodes, as the result of uncontrollable dissolution and deposition of metal anodes, can degrade the performance of batteries.
- It is challenging to find an electrolyte with all desired properties which include high stability, low volatility, non-toxicity and high oxygen solubility and wide electrochemical window.
- The stability of materials where cathodic reactions take places in metal-air batteries is another main challenge. Carbon materials are mainly used for this application and are unstable above 3.5 V during discharge and charge processes. This chemical instability leads to side reactions which are a major cause for performance loss and reduced cyclability of metal-air batteries.
Performance: In metal–air batteries the positive electrode is carbon–based covering with some precious metals for reacting with oxygen. The other electrode is made of a metal such as zinc, Aluminium, magnesium, and lithium. Since in these batteries, the air is flowing through the cell, they are sometimes categorized as fuel cells. The application of air as a cathode helps in lowering the cost and the weight considerably. The utilization of cheap metals as an anode further assists in lowering the cost. Metal-air batteries with ultra-high energy density, including but not limited to Zn-air and Li-air batteries, have shown great potential for future large-scale applications. Metal-air batteries usually contain four major parts: an air electrode, metal electrode, electrolyte, and separator. Li-air batteries and Zn-air batteries are two types of metal-air batteries that have attracted most attention.
The fundamental working principle of a metal-air battery is to electrochemically reduce the oxygen from the air and oxidize the metal. This forms solid metal oxides that may be recycled. This method allows for substantial reductions in the size and the weight of the battery.
Discharging of metal-air batteries involves a few steps. Metal changes into ions on the anodic electrode while oxygen transforms into hydroxide ions at the cathodic electrode. The diffusion of oxygen into the metal-air battery occurs through a layer known as the gas diffusion layer. During the transition of metal into metallic ions, electrons are produced. Metallic ions subsequently dissolve into the electrolyte.
International Advanced Research Centre for Powder Metallurgy and New Materials (ARCI), an autonomous R&D Centre of Department of Science and Technology, Govt. of India, has developed the cost-effective electrocatalyst by anchoring transition metal ions into the sulfur-doped carbon framework via carbonization of a polymer called sPEEK (sulphonated polyether ether ketone). This catalyst synthesis method can also be used to recycle used ionomers (polymer composed of both neutral repeating units and ionized units).
Commercialization: The first primary zinc-air battery was designed by L. Maiche dating back to 1878; its commercial products started to enter market in 1932.
Indian Oil Corporation and Israeli battery developer Phinergy have formalised a joint venture to manufacture ultra-lightweight metal-air batteries for electric vehicles. The joint venture will set up a factory in India to manufacture Aluminium-air batteries for electric vehicles and stationary applications.
Use Cases: Electric cars; Energy storage devices; Water desalination
Patent: US3518123A
Theme: Battery Technology | Subtheme: Metal-air batteries
Source:
Introduction—general features of metal-air batteries, Editor(s): Hajime Arai, Jürgen Garche, Luis Colmenares, Electrochemical Power Sources: Fundamentals, Systems, and Applications, Elsevier 2021
Materials design for rechargeable metal-air batteries. Matter 2019, 1
Metal-Air Batteries: Promises and Challenges, Stanford University 2016
ARCI develops cost-effective catalysts for metal-air battery, Department of Science and Technology, Government of India 2019
IOC, Israeli firm form JV to to manufacture metal-air batteries for EVs, Business Standard, 17 March 2021
Related Documents
Technologies
Long Range Metal-air Battery Technology
Published Year:
Abstract:
A metal-air battery uses metal as an anode just like a li-ion battery, but oxygen drawn in fro... Read More



